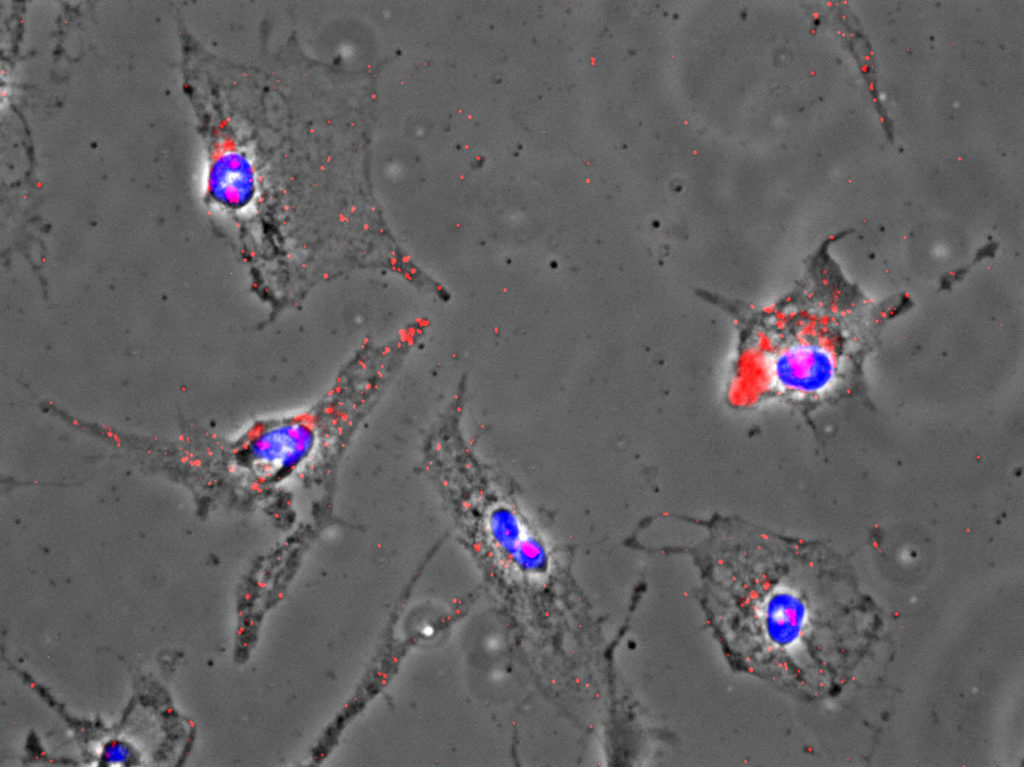
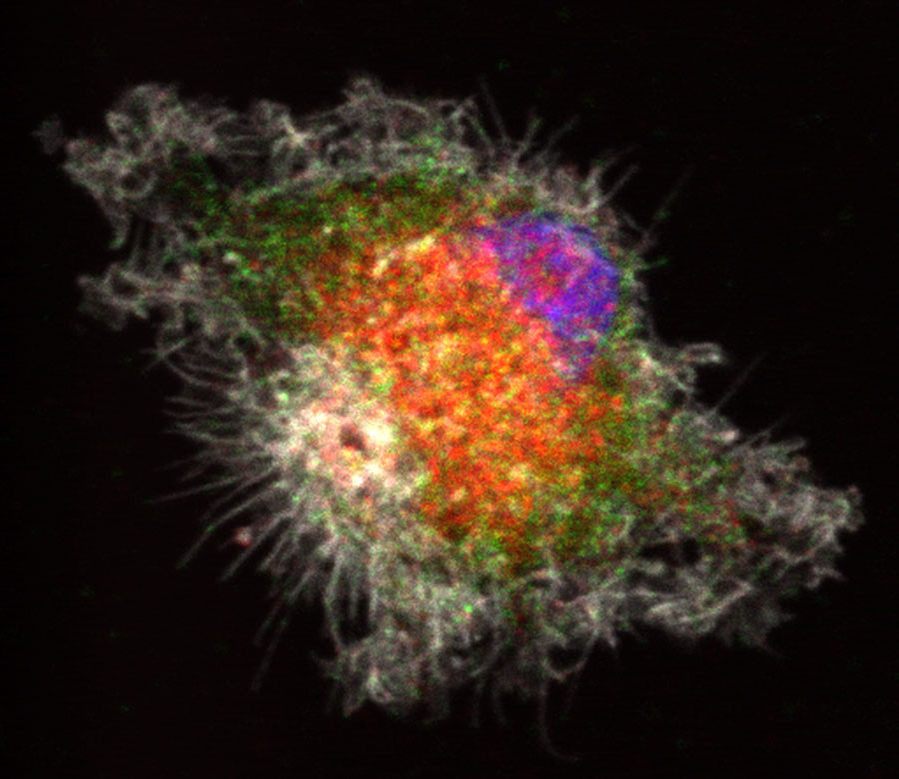
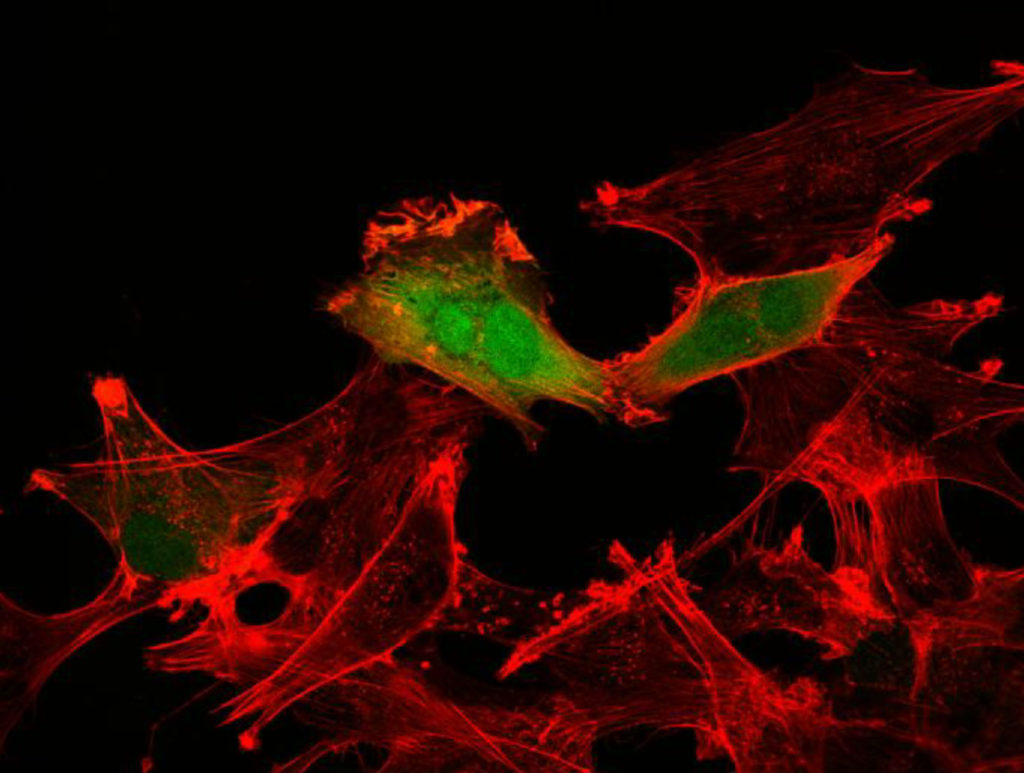
One of the most stark and sobering cancer statistics is that more than 90 percent of the deaths from the disease are the result of metastasis, a process by which the cancer cells spread from the original tumor to migrate through the body to colonize and destroy other organs.
While this morbid statistic is all too familiar to cancer researchers, the process of how tumor cells invade and metastasize to other locations in the body remains poorly understood.
In an effort to unlock this deadly mystery and to help increase cancer survival rates, Dr. Joe W. Ramos and his colleagues at the University of Hawai‘i Cancer Center (UH Cancer Center) have committed themselves to illuminating the molecular mechanisms that drive this process.
“If we can identify exactly how cancer cells move throughout the body we could better develop ways to target these specific mechanisms,” said Ramos, deputy director of the UH Cancer Center. “That could lead to new therapies that block the spread and recurrence of deadly tumors and greatly reduce the number of cancer deaths.”
The spread of cancer by metastasis is driven by a set of mutant proteins called oncogenes, which cause cancer cells to multiply uncontrollably and promotes their ability to move. How oncogene activity specifically directs the increased movement and metastasis is highly complex and remains largely unknown. Ramos and his team have been investigating how these oncogenes and related signals trigger the impairment of normal regulatory processes within the cell and activate the highly mobile and invasive cancer cell behavior. Their work has recently centered on one protein that is turned on by these oncogenes called RSK2 and how this protein drives the aggressive spread of brain cancers throughout the brain.
Brain cancers were specifically targeted by Ramos’ team because it is one of the most aggressive and deadly forms of cancer and can affect any age group. “Brain cancer patients with the most aggressive form, known as glioblastoma multiforme, have only a 15-month median survival time,” said Ramos. “It is therefore imperative to identify new approaches to specifically attack brain cancer cell survival
and motility.”
Ramos and his team have found that the RSK2 protein is highly expressed and active in many patients with brain cancer. The protein propels brain tumor cells into surrounding healthy brain tissue. The invasion of these cells throughout the brain makes it difficult to remove the tumor by surgery, which contributes to a high probability of recurrence and poor survival rates in patients. Additionally, the invading glioblastoma cells are less sensitive to current standard therapies. The research team found that blocking RSK2 stops the invasion of the tumor cells and enhances the effectiveness of standard chemotherapy in tumor cells obtained from patients.
For these tumor cells to move, they must reach forward and grab onto other cells or proteins in the brain and pull. They must also let go at the rear to move forward. Over the course of several years, the team discovered that one of RSK2’s major roles is to orchestrate these movements. In particular, RSK2 instructs proteins at the rear of the cell (integrins) to let go and tells another group of proteins (Rho) to pull up the tail. This is all very carefully coordinated by RSK2 through its ability to alter the charge on the surface of other proteins through a process called phosphorylation. Ramos found that RSK2 phosphorylates the protein filamin A to turn off the integrin adhesion proteins. At the same time, RSK2 phosphorylates a protein called LARG to tell the Rho proteins to contract the cell skeleton to lift up the “tail” of the tumor cells. As a result, the RSK2 protein forms a signaling hub and the oncogenes turn on this signaling hub to activate an invasion and metastasis program. The RSK2 signaling hub has also been found to be active in other cancer types such as skin and breast cancers.
“These results significantly advance our understanding of how cancer cells are made to move during metastasis and suggest more precise targets for drugs to stop cancer metastasis in patients where there are specific oncogenic mutations,” added Ramos. “I’d like to acknowledge Michelle Matter of the UH Cancer Center, Santosh Kesari at the John Wayne Cancer Institute and Dirk Geerts of Erasmus University Medical Center in the Netherlands for contributing to the success of this impactful study.”
Cancer invasion and drug resistance are frequently connected processes and anti-invasion drugs show promise as co-therapeutics with chemotherapy in treatment of brain cancer to prevent recurrence. This work paves the way for the development of new brain cancer therapies focused on blocking the RSK2 function. Currently, Ramos and his team have set their sights on developing new compounds to target this signaling hub. The next steps include identifying better compounds to target the RSK2 protein and testing them in pre-clinical models.
“This research can potentially lead to a new class of drugs to treat not only brain cancers, but other invasive cancers as well,” said UH Cancer Center Director Randall Holcombe. “With about 60 new cases of brain cancer diagnosed every year in Hawai‘i, and about 40 deaths, an effective treatment can help many patients in our state.”